Unveiling the solubility rules worksheet answer key, we embark on a journey into the fascinating realm of ionic compounds, where the ability to predict their solubility is a cornerstone of chemistry. This guide provides a comprehensive understanding of the rules that govern the solubility of ionic compounds, empowering you to confidently navigate the intricacies of this essential chemical concept.
Through a systematic exploration of common solubility rules, exceptions, and practical applications, we unravel the secrets of predicting the solubility of ionic compounds, enabling you to master this fundamental aspect of chemistry.
Definition of Solubility Rules
Solubility rules are a set of guidelines that help predict the solubility of ionic compounds in water. They are based on the principle that certain ions are more likely to form soluble compounds than others. By understanding these rules, chemists can quickly and easily determine whether a given ionic compound will dissolve in water or not.
Common Solubility Rules
The following are the most common solubility rules:Cations
- All Group 1 cations (Li+, Na+, K+, Rb+, Cs+) are soluble.
- All Group 2 cations (Ca2+, Sr2+, Ba2+) are soluble, except for BaSO4.
- All ammonium (NH4+) cations are soluble.
- All cations of metals in Groups 13-15 are insoluble, except for Al3+ and Cr3+.
Anions
- All chloride (Cl-) anions are soluble.
- All bromide (Br-) anions are soluble.
- All iodide (I-) anions are soluble.
- All sulfate (SO42-) anions are soluble, except for BaSO4 and SrSO4.
- All nitrate (NO3-) anions are soluble.
- All carbonate (CO32-) anions are insoluble, except for Na2CO3, K2CO3, and CaCO3.
- All phosphate (PO43-) anions are insoluble, except for Na3PO4, K3PO4, NH43PO4, and the salts of the Group 1 cations.
- All hydroxide (OH-) anions are insoluble, except for the salts of the Group 1 cations.
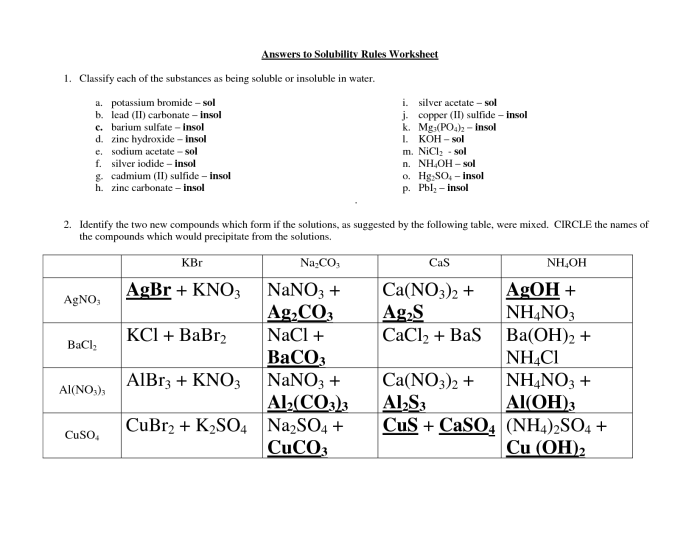
Application of Solubility Rules: Solubility Rules Worksheet Answer Key
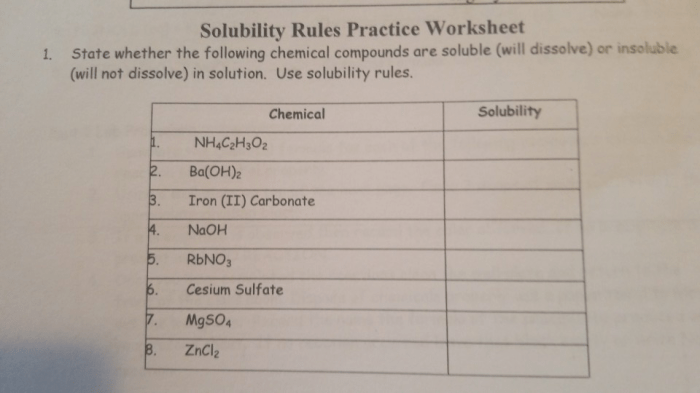
To apply the solubility rules, simply identify the cations and anions present in the ionic compound. Then, check the solubility rules for each ion. If all of the ions are soluble, then the compound is soluble. If any of the ions are insoluble, then the compound is insoluble.For
example, consider the ionic compound NaCl. The cation is Na+, which is soluble according to the solubility rules. The anion is Cl-, which is also soluble according to the solubility rules. Therefore, NaCl is soluble in water.Now consider the ionic compound BaSO4.
The cation is Ba2+, which is soluble according to the solubility rules. However, the anion is SO42-, which is insoluble according to the solubility rules. Therefore, BaSO4 is insoluble in water.
Exceptions to Solubility Rules

There are a few exceptions to the solubility rules. These exceptions are due to the formation of complex ions or the presence of other factors that affect the solubility of the compound.Some common exceptions to the solubility rules include:* BaSO4 is insoluble in water, even though both Ba2+ and SO42- are soluble ions.
This is due to the formation of a complex ion, BaSO42-.
- SrSO4 is insoluble in water, even though Sr2+ and SO42- are both soluble ions. This is also due to the formation of a complex ion, SrSO42-.
- AgCl is insoluble in water, even though Ag+ and Cl- are both soluble ions. This is due to the formation of a complex ion, AgCl2-.
- PbCl2 is insoluble in water, even though Pb2+ and Cl- are both soluble ions. This is due to the formation of a complex ion, PbCl42-.
Clarifying Questions
What are the most common solubility rules?
The most common solubility rules include the following: – All Group 1 cations (Li+, Na+, K+, Rb+, Cs+) are soluble. – All Group 2 cations (Ca2+, Sr2+, Ba2+) are soluble, except for BaSO4. – All ammonium (NH4+) cations are soluble.
– All nitrate (NO3-) anions are soluble. – All chloride (Cl-) anions are soluble, except for AgCl, PbCl2, and Hg2Cl2. – All bromide (Br-) anions are soluble, except for AgBr, PbBr2, and Hg2Br2. – All iodide (I-) anions are soluble, except for AgI, PbI2, and Hg2I2.
– All sulfate (SO42-) anions are soluble, except for BaSO4 and SrSO4.
What are some exceptions to the solubility rules?
Some exceptions to the solubility rules include the following: – BaSO4 is insoluble. – SrSO4 is slightly soluble. – AgCl, PbCl2, and Hg2Cl2 are insoluble. – AgBr, PbBr2, and Hg2Br2 are slightly soluble. – AgI, PbI2, and Hg2I2 are insoluble.
How can I use solubility rules to predict the solubility of an ionic compound?
To use solubility rules to predict the solubility of an ionic compound, follow these steps: 1. Identify the cation and anion of the compound. 2. Check the solubility rules for the cation and anion. 3. If both the cation and anion are soluble, then the compound is soluble.
4. If either the cation or anion is insoluble, then the compound is insoluble.