Physical chemical changes & properties color by number – Embark on a captivating journey into the realm of physical chemical changes and properties, where the vibrant hues of color unveil the intricate secrets of matter. This exploration unveils the fundamental distinctions between physical and chemical transformations, delving into the defining characteristics of each.
Prepare to unravel the mysteries of matter’s properties, discovering how physical and chemical attributes shape the world around us. Through the engaging medium of color by number, we’ll witness firsthand how color serves as a powerful tool in identifying and understanding the diverse properties of matter.
As we delve deeper, we’ll uncover the profound relationship between color and chemical structure, revealing the intricate interplay between the molecular makeup of substances and their visual appearance. This captivating exploration promises to illuminate the fascinating world of physical chemical changes and properties, leaving you with a newfound appreciation for the colorful tapestry of matter.
Physical and Chemical Changes: Physical Chemical Changes & Properties Color By Number
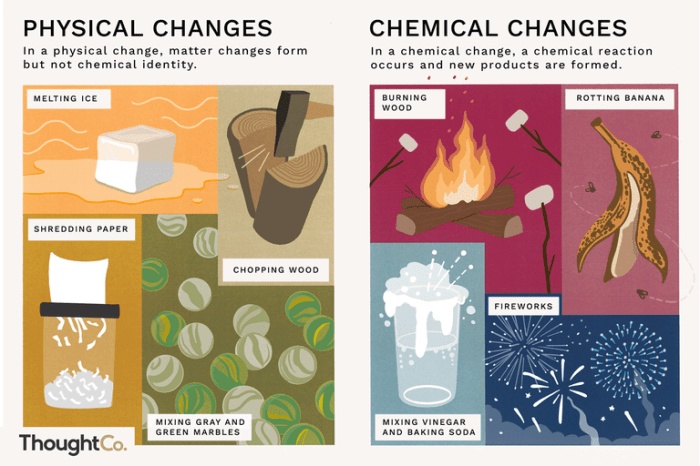
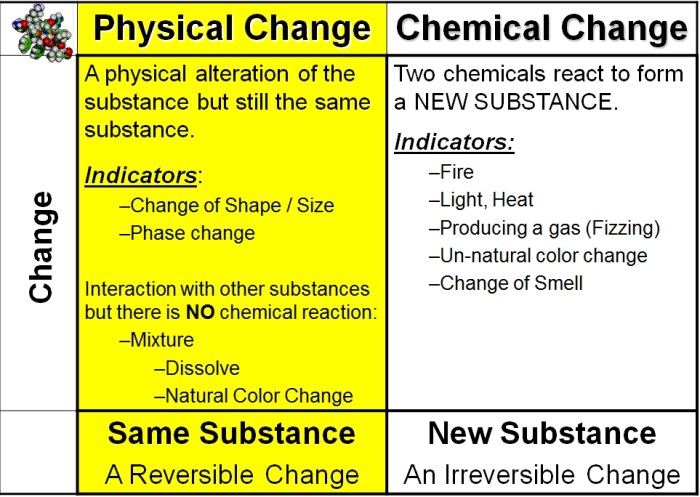
Matter can undergo changes that can be classified as either physical or chemical. A physical change alters the form or appearance of a substance without changing its chemical composition. Examples of physical changes include melting, freezing, boiling, sublimation, and condensation.
A chemical change, on the other hand, involves a change in the chemical composition of a substance. Examples of chemical changes include burning, rusting, and cooking.
Identifying Physical and Chemical Changes
Physical changes are typically reversible, meaning that the original substance can be restored by reversing the change. Chemical changes are usually irreversible, meaning that the original substance cannot be restored by reversing the change.
Properties of Matter
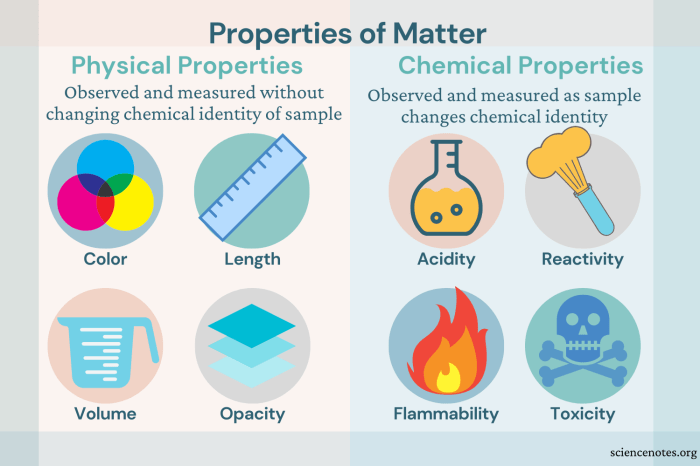
The properties of matter are characteristics that describe its physical and chemical behavior. Physical properties include color, density, solubility, and melting point. Chemical properties describe how a substance reacts with other substances. Examples of chemical properties include flammability, reactivity, and toxicity.
Physical and Chemical Properties
Physical properties can be observed without changing the chemical composition of a substance. Chemical properties, on the other hand, can only be observed when a substance undergoes a chemical change.
Color by Number
Color by number is an activity in which a person fills in areas of a design with specific colors based on numbers assigned to each area. This activity can be used to create simple or complex designs, and it can be enjoyed by people of all ages.
Benefits of Color by Number Activities
Color by number activities can provide a number of benefits, including:
- Improved fine motor skills
- Enhanced color recognition
- Increased attention to detail
- Reduced stress and anxiety
Color and Properties
Color can be used to identify different properties of matter. For example, the color of a substance can indicate its chemical composition, its physical state, or its temperature. The relationship between color and chemical structure is a complex one, but it is well-known that certain functional groups tend to produce certain colors.
Using Color to Identify Substances, Physical chemical changes & properties color by number
The color of a substance can be used to identify it. For example, copper is a reddish-brown metal, and gold is a yellow metal. The color of a substance can also change when it undergoes a chemical change. For example, iron turns red when it rusts.
Questions Often Asked
What is the key difference between physical and chemical changes?
Physical changes alter the form or appearance of a substance without modifying its chemical composition, while chemical changes involve the rearrangement of atoms to form new substances with distinct properties.
How can color be used to identify different substances?
Color arises from the interaction of light with matter, and different substances exhibit unique absorption and reflection patterns, allowing us to use color as a tool for identification.
What are the benefits of using color by number activities in education?
Color by number activities engage students, enhance their understanding of concepts, and foster creativity while reinforcing problem-solving skills.