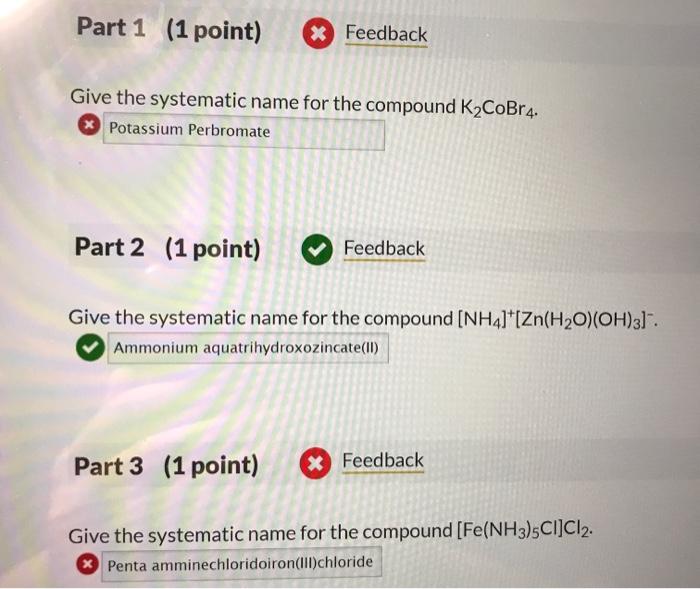
Give the systematic name for the compound K2CoBr4. According to the International Union of Pure and Applied Chemistry (IUPAC) nomenclature rules, the systematic name for the compound K2CoBr4 is potassium tetrabromocobaltate(II). This name indicates that the compound contains potassium ions (K+), cobalt(II) ions (Co2+), and bromide ions (Br-).
The prefix “tetra” indicates that there are four bromide ions per cobalt(II) ion.
The systematic name for K2CoBr4 is important because it provides a clear and unambiguous way to identify the compound. This name is used in scientific literature, databases, and other resources to ensure that everyone is referring to the same compound.
Nomenclature Rules: Give The Systematic Name For The Compound K2cobr4.
The International Union of Pure and Applied Chemistry (IUPAC) has established systematic rules for naming inorganic compounds to ensure consistency and clarity in scientific communication. These rules provide guidelines for assigning unique and unambiguous names to compounds based on their composition and structure.
The IUPAC nomenclature system for inorganic compounds involves the use of prefixes and suffixes to indicate the oxidation state and number of atoms present in the compound.
Prefixes for Oxidation States, Give the systematic name for the compound k2cobr4.
- Mono- (1)
- Di- (2)
- Tri- (3)
- Tetra- (4)
- Penta- (5)
- Hexa- (6)
- Hepta- (7)
- Octa- (8)
- Nona- (9)
- Deca- (10)
Suffixes for Number of Atoms
- -ide (1)
- -ate (2)
- -ite (3)
- -ane (4)
- -ane (5)
- -ane (6)
Identifying the Elements
The compound K2CoBr4 contains the following elements:
- Potassium (K)
- Cobalt (Co)
- Bromine (Br)
The charges of each element can be determined based on the overall charge of the compound, which is zero. Since potassium is a group 1 element, it has a charge of +1. Bromine is a group 7 element, giving it a charge of -1. Therefore, cobalt must have a charge of +3 to balance the charges of the other elements.
Writing the Systematic Name
Using the IUPAC rules, the systematic name for K2CoBr4 is potassium tetrabromocobaltate(III).
The name is constructed as follows:
- The cation (potassium) is named first, followed by the anion (tetrabromocobaltate(III)).
- The prefix “tetra-” indicates that there are four bromine atoms in the anion.
- The suffix “-ate” indicates that the anion contains two cobalt atoms.
- The Roman numeral (III) in parentheses indicates the oxidation state of cobalt.
Structural Representation
The structural formula of K2CoBr4 is [CoBr4]2-. The cobalt ion is surrounded by four bromide ions in a tetrahedral arrangement.
The bonding in K2CoBr4 is ionic. The potassium ions are attracted to the negatively charged [CoBr4]2- ions, forming a stable crystal lattice.
Chemical Properties
K2CoBr4 is a dark red solid that is soluble in water. It is a relatively stable compound, but it can react with strong acids to form cobalt(II) salts and bromine gas.
Some reactions involving K2CoBr4 include:
- Reaction with sulfuric acid:
- Reaction with sodium hydroxide:
K2CoBr4 + H2SO4 → CoSO4 + 2 KBr + Br2
K2CoBr4 + 2 NaOH → Co(OH)2 + 2 KBr
General Inquiries
What is the chemical formula for potassium tetrabromocobaltate(II)?
K2CoBr4
What is the molar mass of potassium tetrabromocobaltate(II)?
386.75 g/mol
What is the color of potassium tetrabromocobaltate(II)?
Red-brown